Taking part in the POSNOC trial

What will happen if you take part?
You should look at all the information on this website and talk to your doctors to discuss this study before you decide to take part. The patient information leaflet and patient information videos will help you to fully understand how the study will be done and what will happen if you decide to take part in the study. Your care and recovery are most important to us and will not be affected if you choose not to take part in the study. There are no extra tests or procedures in the study.
You will be asked to sign a consent form before you join the study and complete short questionnaires which ask about how you are, and any problems in your arm and hand. Once you have registered you will be randomly placed into one of two groups. There won’t be a need for any extra tests or procedures.
You will be followed up in the outpatient clinic six months after you join the study, then at one year, and yearly after that till five years. If you do not have a clinic appointment at that time we may contact you by telephone. During follow-up, we will check for any signs of cancer coming back and ask you to answer a few questions about problems in your arm and hand.
We will post you short questionnaires, along with a stamped self addressed envelope, that ask about your quality of life, your ability to carry out your normal daily activities and any feelings of anxiety you have at three months, six months, one year, two years and three years. These questionnaires are quite short and extremely important for the study.
If you decide to take part in the study, you are free to withdraw at any time, without giving a reason. This will not affect the standard of care you receive.
What are the differences between the two treatment groups in the study?
There are two treatment groups in the study. In both groups women will receive drug therapy which can be chemotherapy, endocrine therapy (hormone therapy), or both and radiotherapy to the breast or chest wall if needed. The only difference between the groups is that women in one group will undergo armpit treatment (axillary treatment) and women in the second group will not. Armpit treatment is either a second operation to remove all the remaining lymph glands in the armpit (axillary node clearance) or radiotherapy to the armpit (axillary radiotherapy) depending on local hospital practice.
If you choose to be part in POSNOC you will be randomly placed in one of the two groups. Neither you nor your doctor or nurse will be able to choose which group you are allocated to. This is done so that we can make a fair comparison with equal numbers of patients of different ages and state of health in each group. At the end of the study we need to be sure that any differences between women in the two groups are due to whether or not they had armpit treatment, rather than anything else.
You should only take part if you are comfortable being randomly placed into either of the two treatment groups. Please see below a helpful diagram of standard care vs taking part in the POSNOC trial.
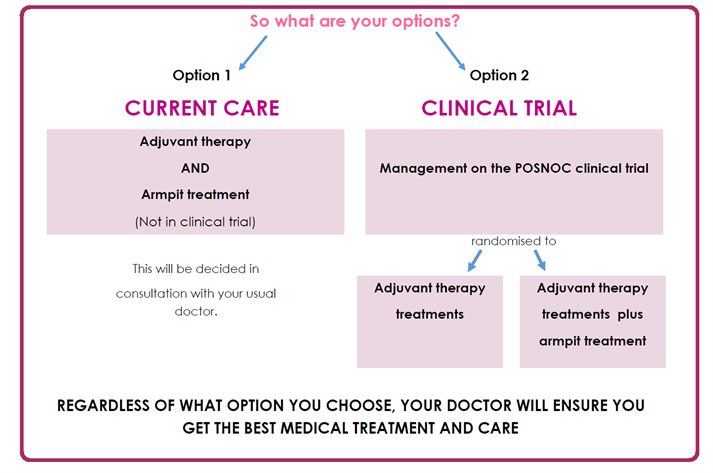
Risks and benefits of taking part
We do not know whether armpit treatment has any effect on the chance of your cancer coming back. That is why we are doing this study.
You will have regular examinations, regardless of which group you are in, to check is the cancer is coming back. Your doctor will discuss with you the best course of action if you are found to have armpit recurrence.
We cannot promise the study will help you. But information we get from the study will help improve future care for patients with early breast cancer that has spread to one or two lymph glands.
What happens after the study
Normal NHS cancer care is that patients are discharged from the hospital clinic after five years. So when you have finished the study you will probably be discharged from the clinic, but this decision will be made by your clinical team.
If at any time during the study you decide not to take part in the follow up assessments or not to complete the sets of questionnaires, we will use the data collected up to that point, unless you ask us not to.
If the evidence shows that there is an important difference between the two forms of care for women, then we may want to contact you again in the future to find out how you are.
Will my taking part be kept confidential?
Yes. All information we collect about you will be kept in the strictest confidence. In order to be able to contact you to find out how you have been since your treatment, your name and contact details will be made available to the researchers running this study and held at the co-ordinating centres, Nottingham CTU and SHORE-C, and not just by your local study doctor. These details will be kept securely, with restricted access. You will not be named or otherwise identified in any study publication.
Further reading
There is lots of useful breast cancer information on the Breast Cancer Care and Macmillan websites
